4 | Structure of Atoms
Structure of Atoms
- Atoms are made up of three main subatomic particles – protons, neutrons and electrons.
- Protons and neutrons form a cluster at the centre of the atom, called the nucleus.
- Electrons orbit the nucleus in electron shells, forming an electron cloud.
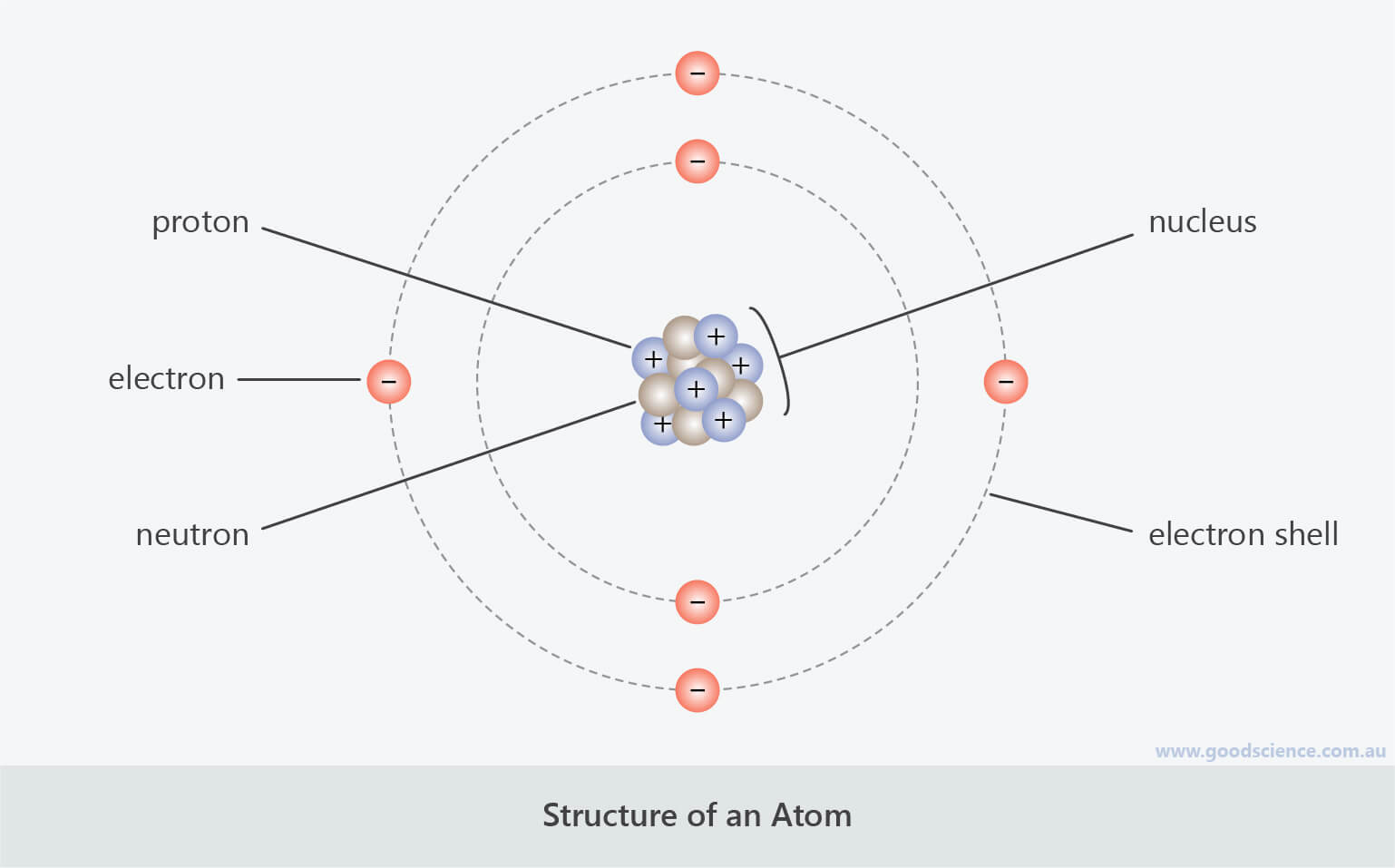
Atoms are made up of protons, neutrons and electrons.
Protons
- Protons are subatomic particles with a positive charge (+1).
- Protons have a mass of 1 atomic mass unit.
- The number of protons in an atom determines the type of atom.
- Examples
- If an atom has 2 protons, it is a helium atom.
- If an atom has 6 protons, it is a carbon atom.
- If an atom has 26 protons, it is an iron atom.
- The number of protons in an atom is known as the atomic number.

Neutrons
- Neutrons are subatomic particles with no charge.
- Neutrons have a mass of 1 atomic mass unit.
- The number of neutrons in a particular type of atom can vary.
- For example, 99% of carbon atoms have 6 neutrons, while 1% have 7 neutrons.
- The total number of protons and neutrons in an atom is known as the mass number.

- Atoms that have the same number of protons but a different number of neutrons are called isotopes.
- Therefore, isotopes of an element have the same atomic number, but different mass numbers.
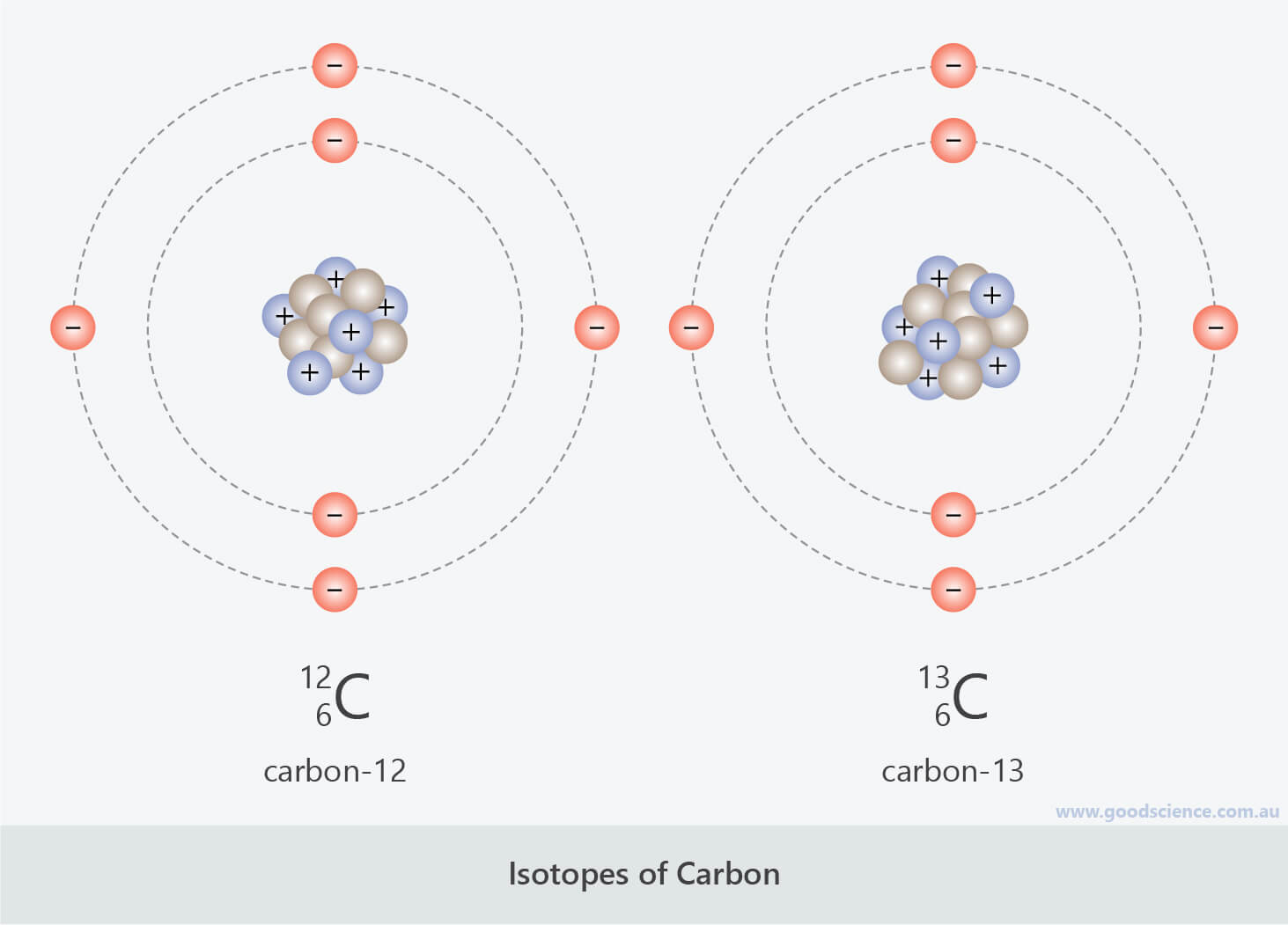
Carbon-12 and carbon-13 are isotopes of carbon.
Electrons
- Electrons are subatomic particles with a negative charge (–1).
- Electrons have a mass of 1/1840 of an atomic mass unit.
- Therefore, almost all of the mass of an atom is in the nucleus.
- The number of electrons in an atom equals the number of protons.
- Therefore, atoms have no overall charge.

Quizzes