3 | Atoms, Molecules and Lattices
Chemical Subunits: Atoms, Molecules and Lattices
- A chemical subunit can be defined as the smallest building block of a pure substance that is unique to that substance.
- Atoms are the simplest type of chemical subunit, forming the building blocks of all matter.
- Atoms can exist individually, but more often they are connected to other atoms by chemical bonds, forming molecules or lattices.
- Molecules are discrete arrangements of atoms joined by chemical bonds.
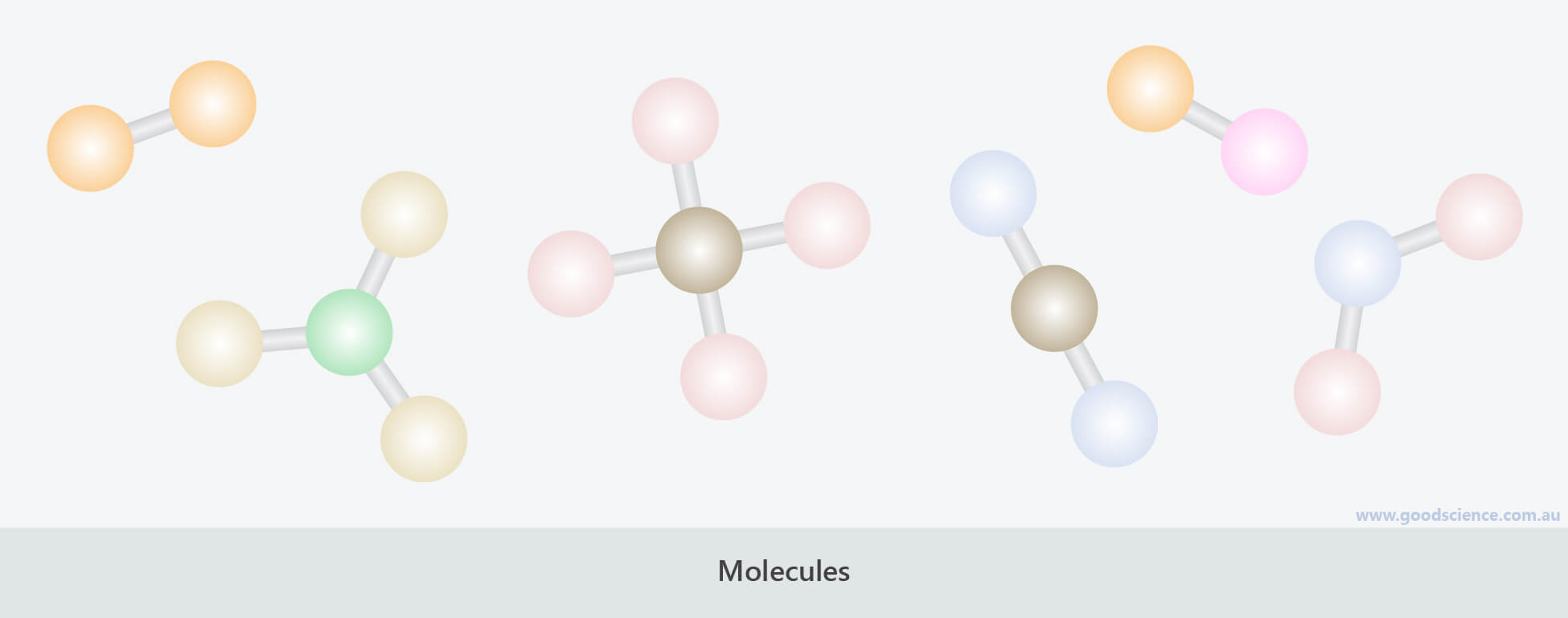
Molecules are discrete structures held together by chemical bonds.
- Lattices are networks of atoms joined by chemical bonds.
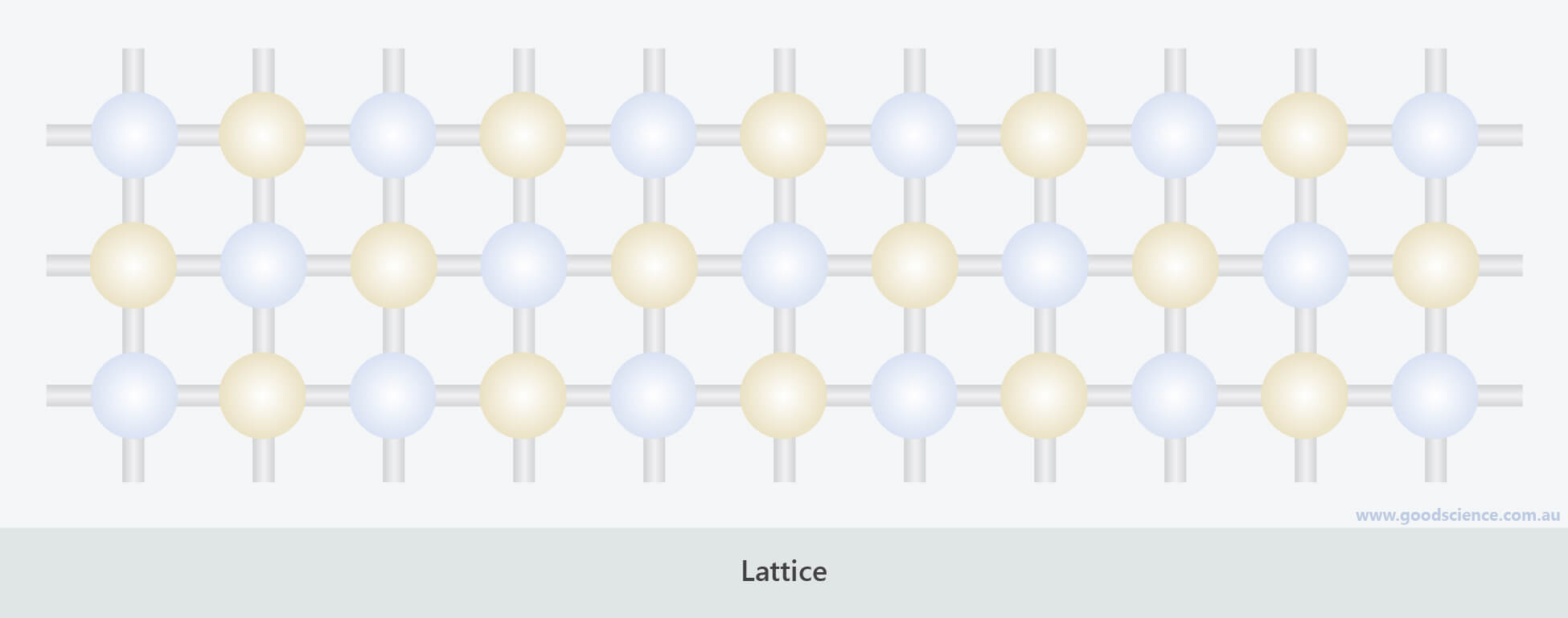
Lattices are continuous structures held together by chemical bonds.
- Molecules and lattices may be composed of the same type of atom, or they may be composed of two or more different types of atoms, in fixed arrangements.
Arrangement of Atoms in Pure Substances
- Pure substances have fixed properties because they are made up of one type of chemical subunit.
- Mixtures have variable properties because they are made up of more than one type of chemical subunit.
Arrangement of Atoms in Elements
- Elements are pure substances that cannot be broken down into simpler substances because their subunits consist of one type of atom.
- These atoms may exist individually, or as molecules or lattices.
- Examples
- The atoms in helium exist individually.
- The atoms in oxygen exist as molecules containing pairs of oxygen atoms.
- The atoms in carbon (diamond) exist as a continuous network.


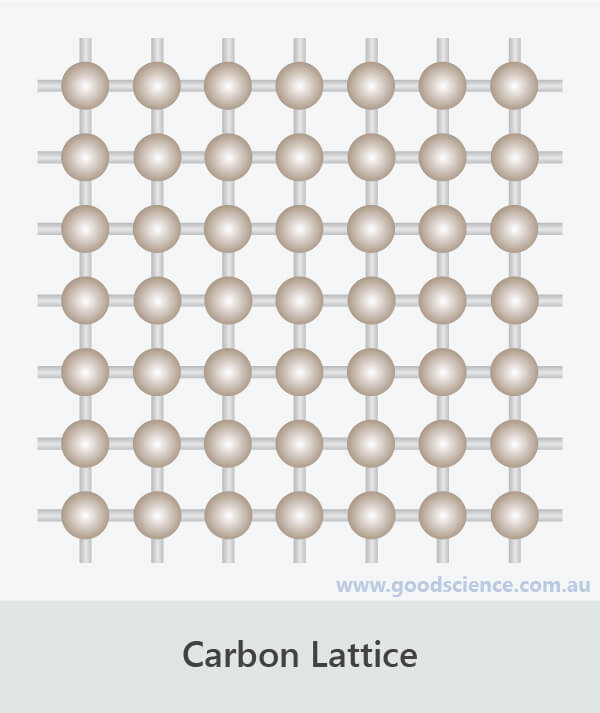
Atoms in elements can exist individually, as molecules or as lattices.
Arrangement of Atoms in Compounds
- Compounds are pure substances that can be broken down into simpler substances because their subunits consist of more than one type of atom.
- These atoms may exist as molecules or lattices.
- Examples
- The atoms in carbon dioxide exist as molecules containing one carbon atom and two oxygen atoms.
- The atoms in silicon dioxide (quartz) exist as continuous networks of silicon and oxygen atoms, in a 1:2 ratio.

![]()
Atoms in compounds can exist as molecules or as lattices.