3 | Solutions, Coarse Mixtures and Colloids
Classification of Mixtures as Solutions, Coarse Mixtures or Colloids
- Although mixtures are generally divided into two categories – homogeneous mixtures and heterogeneous mixtures – this is based on their appearance when viewed with the naked eye.
- However, certain substances, like milk, sunscreen and paint, might appear homogeneous, but when they are viewed under a microscope, separate phases are visible.
- In other words, they don’t have a homogeneous (uniform) composition.

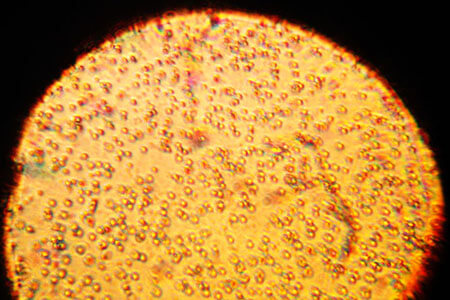
Milk appears homogeneous with the naked eye, but under a microscope separate phases are visible.
(Images: Couleur, Pixabay; Amangeldyurazov, Wikimedia Commons)
- Therefore, whether you are describing the composition of a mixture as homogeneous or heterogeneous depends on whether you are basing this on its appearance when viewed with a naked eye (its macroscopic appearance) or its appearance when viewed under a microscope (its microscopic appearance).
- When considering both the macroscopic and microscopic composition of mixtures, three categories of classifications become necessary: solutions, coarse mixtures and colloids.
Solutions
- Solutions are mixtures that appear homogeneous with the naked eye as well as under a microscope.
- In other words, they are macroscopically and microscopically homogeneous.
- Examples of solutions include the mouthwash and stainless steel mentioned earlier, as well as petrol, methylated spirits and bleach.



Petrol, methylated spirits and bleach are solutions.
(Images: AmericanXplorer13, Wikimedia Commons; Longhair, Wikimedia Commons; MarkGallagher, Wikimedia Commons)
Coarse mixtures
- Coarse mixtures are mixtures that appear heterogeneous with the naked eye as well as under a microscope.
- In other words, they are macroscopically and microscopically heterogeneous.
- Examples of coarse mixtures include the mud and cereal mentioned earlier, as well as smoke, soup and concrete.



Smoke, soup and concrete are coarse mixtures.
(Images: jackmac34, Pixabay; Couleur, Pixabay; Projekt_Kaffeebart, Pixabay)
Colloids
- Colloids are mixtures that appear homogeneous with the naked eye, but heterogeneous under a microscope.
- In other words, they are macroscopically homogeneous, but microscopically heterogeneous.
- Examples of colloids include the milk, sunscreen and paint mentioned earlier, as well as plain yoghurt, moisturiser cream and shaving foam.



Yoghurt, hand cream and shaving foam are colloids.
(Images: Profet77, Pixabay; pxhere; CoffeeAddict, Wikimedia Commons)

