2 | Homogeneous and Heterogeneous Mixtures
Homogeneous and Heterogeneous Mixtures
- Mixtures can be divided into two types based on their composition. These are homogeneous mixtures and heterogeneous mixtures.
Homogeneous Mixtures
- Homogeneous mixtures have a uniform composition.
- This means that regardless of where a sample is taken from, the sample would contain the same blend of components.
- Examples of homogeneous mixtures include mouthwash, paint and stainless steel.



Mouthwash, paint and stainless steel are homogeneous mixtures.
(Images: Erschaffung, Wikimedia Commons; ulleo, Pixabay; RonPorter, Pixabay)
Heterogeneous Mixtures
- Heterogeneous mixtures have a non-uniform composition.
- This means that if samples were taken from different places within the mixture, the blend of components would not be the same.
- Examples of heterogeneous mixtures include mud and a bowl of cereal.



Breakfast cereals, mud and concrete are heterogeneous mixtures.
(Images: ImagesBG, Pixabay; skeeze, Pixabay; US Navy, Wikimedia Commons)
Phases
- A phase is a part of a mixture that has a uniform composition.
- Since homogeneous mixtures have a uniform composition throughout, they have only one phase.
- Since heterogeneous mixtures do not have a uniform composition throughout, they have more than one phase.
- For example, an oil-water mixture has an oil phase and a water phase.
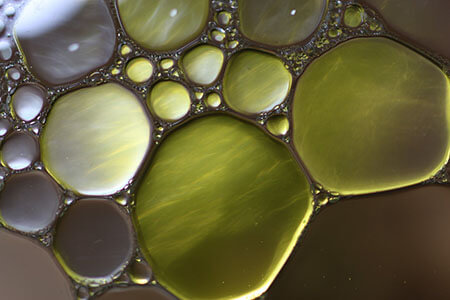
Oil and water form separate phases within a mixture.
(Image: the3cats, Pixabay)

